CDK inhibitor E9
CAS No. 2020052-55-3
CDK inhibitor E9( E9 )
Catalog No. M13145 CAS No. 2020052-55-3
CDK inhibitor E9 is a novel CDK inhibitor that can overcomes ABC-mediated resistance of THZ1, functions as a potent, non-covalent inhibitor of CDK9 and a covalent inhibitor of CDK12.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 299 | Get Quote |


|
| 50MG | 1485 | Get Quote |


|
| 100MG | 2250 | Get Quote |


|
| 200MG | Get Quote | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NameCDK inhibitor E9
-
NoteResearch use only, not for human use.
-
Brief DescriptionCDK inhibitor E9 is a novel CDK inhibitor that can overcomes ABC-mediated resistance of THZ1, functions as a potent, non-covalent inhibitor of CDK9 and a covalent inhibitor of CDK12.
-
DescriptionCDK inhibitor E9 is a novel CDK inhibitor that can overcomes ABC-mediated resistance of THZ1, functions as a potent, non-covalent inhibitor of CDK9 and a covalent inhibitor of CDK12; does not serve as a substrate for either ABCB1 or ABCG2, while avoiding ABC transporter-mediated efflux; decreases the phosphorylated and total RNAPII in THZ1R NB and lung cancer cells (IC50=8-40 nM), accompanied by decreased MYC and MCL1 expression, exerts its cytotoxic effects through covalent modification of cysteine 1039 of CDK12.
-
In VitroCDK12-IN-E9 (E9; 10 nM-10 μM; 72 hours; Kelly, LAN5, SK-N-BE2, PC-9, NCI-H82 and NCI-H3122 cells) treatment shows potent antiproliferative activity in THZ1R NB and lung cancer cells, with IC50 values ranging from 8 to 40 nM.CDK12-IN-E9 (E9; 0-3000 nM; 6 hours; Kelly, PC-9, and NCI-H82 cells) treatment leads to a dose-dependent decrease in phosphorylated and total RNAPII in THZ1r NB and lung cancer models, accompanied by decreased MYC and MCL1 expression.CDK12-IN-E9 also results in increased PARP cleavage, and an increase in the subGI population in THZ1r lung cancer cells, while in NB cells, more of a G2/M arrest is seen after a 24-hr exposure to CDK12-IN-E9. Cell Proliferation Assay Cell Line:Kelly, LAN5, SK-N-BE2, PC-9, NCI-H82 and NCI-H3122 cells Concentration:10 nM-10 μM Incubation Time:72 hours Result:Showed potent antiproliferative activity in THZ1R NB and lung cancer cells, with IC50 values ranging from 8 to 40 nM.Western Blot Analysis Cell Line:Kelly, PC-9, and NCI-H82 cells Concentration:0 nM, 30 nM, 100 nM, 300 nM, 1000 nM, 3000 nM Incubation Time:6 hours Result:Led to a dose-dependent decrease in phosphorylated and total RNAPII in THZ1r NB and lung cancer models.
-
In Vivo——
-
SynonymsE9
-
PathwayAngiogenesis
-
TargetCDK
-
RecptorCDK
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number2020052-55-3
-
Formula Weight434.544
-
Molecular FormulaC24H30N6O2
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 125 mg/mL (287.67 mM)
-
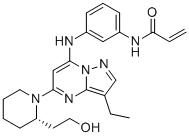
SMILESC=CC(NC1=CC=CC(NC2=CC(N3[C@H](CCO)CCCC3)=NC4=C(CC)C=NN24)=C1)=O
-
Chemical Name(S)-N-(3-((3-ethyl-5-(2-(2-hydroxyethyl)piperidin-1-yl)pyrazolo[1,5-a]pyrimidin-7-yl)amino)phenyl)acrylamide
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference



-
BML-259
BML-259 is an inhibitor of CDK5 and CDK2 with IC50s of 64 and 98 nM, respectively. BML-259 can be used in studies about the treatment of cancer and neurodegenerative diseases.
-
SB415286
SB415286 is a potent GSK3α inhibitor with IC50/Ki of 78 nM/31 nM with equally effective inhibition of GSK-3β.
-
Trilaciclib hydrochl...
Trilaciclib hydrochloride is an inhibitor of CDK4/6 (IC50s: 1 nM and 4 nM for CDK4 and CDK6).Incubation with Trilaciclib hydrochloride (G1T28) for 24 hours can induce a strong G1 cell cycle arrest (time=0). Cells have re-entered the cell cycle and demonstrate cell-cycle kinetics similar to untreated control cells By 16 hours after Trilaciclib hydrochloride washout.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


